Services
Management System Certification
- GDPMD
- ISO 9001
- ISO 14001
- ISO 45001
- ISO 13485
GDPMD specifies the requirements for a quality management system to be established, implemented and maintained by an establishment in carrying out activities in medical device supply-chain to comply with regulatory requirements. GDPMD requires an establishment to demonstrate its ability to maintain quality, safety and performance of medical devices in compliance with the medical device regulatory requirement throughout the supply-chain. It shall be used by both the internal and external parties to determine the ability of an establishment to meet the requirements specified within.
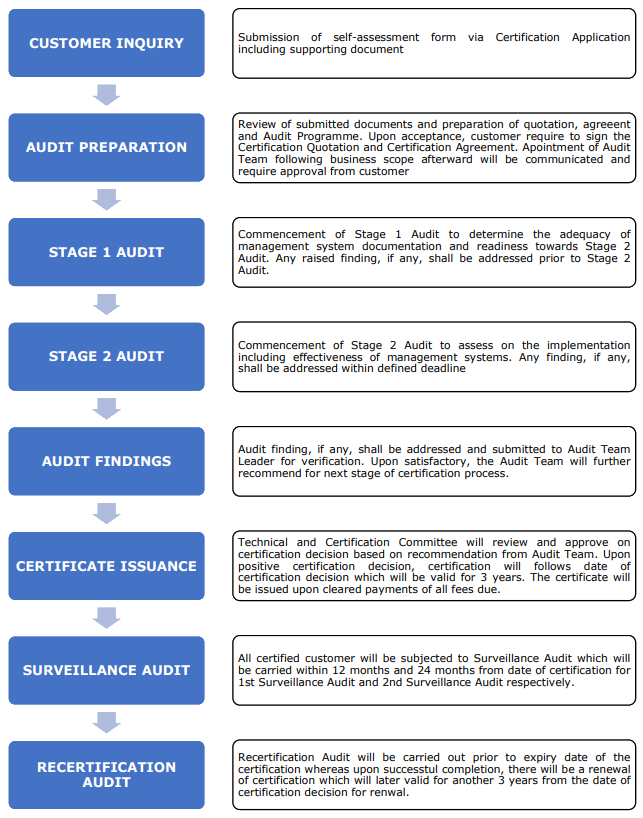
Kindly contact us further at [email protected] for further information.
ISO 9001 is an international standard that sets out the criteria for a quality management system (QMS). It provides a framework for organizations to establish, implement, maintain, and continually improve their quality management processes. ISO 9001 is used by businesses and organizations of all sizes and industries to demonstrate their commitment to delivering quality products and services.

Kindly contact us further at [email protected] for further information.
ISO 14001 is an internationally recognized standard that sets out the criteria for an environmental management system (EMS). It helps organizations of all sizes and industries to systematically manage and reduce their environmental impact, comply with environmental regulations, and demonstrate a commitment to environmental sustainability.

Kindly contact us further at [email protected] for further information.
ISO 45001 is an international standard that specifies requirements for an occupational health & safety (OHS) management system. It provides a framework for organizations to manage risks and opportunities to help prevent worker illnesses and injuries.

Kindly contact us further at [email protected] for further information.
ISO 13485 is an international standard that specifies the requirements for a quality management system specific to the medical device industry. It outlines the processes and procedures necessary to ensure the quality, safety, and effectiveness of medical devices throughout their lifecycle, from design and development to production, distribution, and post-market surveillance.

Kindly contact us further at [email protected] for further information.
