FAQ
- Management System Certification
- Medical Device Conformity Assessment
- Appeal
- Complaint
What is GDPMD?
GDPMD, or Good Distribution Practice for Medical Devices, is a set of regulations and standards that outline the requirements for the proper distribution and supply chain management of medical devices in Malaysia. It is designed to ensure the quality, safety, and integrity of medical devices as they move through the distribution process, from manufacturers to end-users or healthcare facilities.
What are the benefits of GDPMD?
The benefits of adhering to GDPMD include:
- Quality Assurance: GDPMD helps maintain the quality and integrity of medical devices during storage, transport, and distribution.
- Compliance: It ensures compliance with Malaysia’s Medical Device Act 2012 and Regulations 2012, which are crucial for organizations involved in the distribution of medical devices.
- Patient Safety: Proper distribution practices help ensure that medical devices reach end-users in safe and effective conditions, contributing to patient safety.
- Minimized Risk: GDPMD guidelines reduce the risk of counterfeit or substandard medical devices entering the market.
- Legal Requirements: Compliance with GDPMD is a legal requirement for entities engaged in the distribution of medical devices in Malaysia.
Who shall apply GDPMD Certification?
GDPMD certification is typically applicable to entities involved in the distribution and supply chain management of medical devices in Malaysia. This includes:
- Authorized Representatives: Companies appointed by a manufacturer having a principal place of business outside Malaysia.
- Distributors: Companies or individuals that distribute medical devices to healthcare providers, pharmacies, or other end-users.
- Importers: Entities that import medical devices into Malaysia for distribution.
- Wholesalers and Retailers: Businesses engaged in the wholesale or retail distribution of medical devices.
- Logistics and Storage Providers: Companies that provide storage and transportation services for medical devices.
In the context of compliance against Section 15 of Medical Device Act 2012 by Authorized Representatives, Importers and Distributors, GDPMD certification is a mandatory and act as pre-requisite requirement to apply for Establishment License for respective role.
What is the involved certification process to be certified with GDPMD?
The certification process based on Medical Device Act 2012, Medical Device Regulations 2012 and ISO/IEC 17021-1 requirement which involves the following steps:
- Certification Application: Authorized representative of the company require to fill up Certification Application for Application Review purpose and further preparation of Quotation and Certification Agreement.
- Quotation and Certification Agreement: Upon acceptance on the terms and conditions, LEADER will proceed with the preparation of Audit Programme and planning of audit activities.
- Stage 1 Audit: The 1st stage of Initial Certification Audit to determine the adequacy of the client’s management system documentation and readiness to proceed to the Stage 2 audit.
- Stage 2 Audit: The 2nd stage of Initial Certification Audit to evaluate the implementation, including effectiveness, of the client’s management system.
- Recommendation: Upon successful completion of audit activities, Audit Team Leader will recommend to Technical & Certification Committee for further certification process
- Certification Issuance: Technical & Certification Committee will review and approve the certification application based on respective standard requirement. Upon satisfaction, GDPMD Certificate will be granted.
- Surveillance Audit: Annual audit based on respective standard to ensure ongoing compliance.
- Recertification Audit: Renewal of certification is subject to a satisfactory recertification audit carry out before the expiry of the certificate.
How long does it take to be certified with GDPMD?
The time required for certification can vary widely depending on the size and complexity of the organization, its existing processes, and its commitment to meeting GDPMD requirements.
Kindly contact us further at admin@leadercb.com for further information.
How much the cost of GDPMD certification?
The cost of GDPMD certification varies depending on factors such as the size and complexity of the organization, scope of activities and the location.
Kindly contact us further at admin@leadercb.com for further information.
I am already certified with GDPMD by another Conformity Assessment Body, can I transfer my certification to LEADER?
Yes, it is possible.
Kindly contact us further at admin@leadercb.com for further information.
What is ISO 9001?
ISO 9001 is an international standard that sets out the criteria for a quality management system (QMS). It provides a framework for organizations to establish, implement, maintain, and continually improve their quality management processes. ISO 9001 is used by businesses and organizations of all sizes and industries to demonstrate their commitment to delivering quality products and services.
What are the benefits of ISO 9001?
The benefits of implementing ISO 9001 include:
- Improved product or service quality
- Enhanced customer satisfaction
- Increased efficiency and productivity
- Better management of resources
- Greater consistency in processes
- Improved decision-making
- Competitive advantage
- Compliance with legal and regulatory requirements
Who shall apply ISO 9001 Certification?
Any organization, regardless of its size or industry, can apply for ISO 9001 certification if it wants to establish and demonstrate a robust quality management system.
What is the involved certification process to be certified with ISO 9001?
The certification process based on ISO/IEC 17021-1 requirement involves the following steps:
- Certification Application: Authorized representative of the company require to fill up Certification Application for Application Review purpose and further preparation of Quotation and Certification Agreement
- Quotation and Certification Agreement: Upon acceptance on the terms and conditions, LEADER will proceed with the preparation of Audit Programme and planning of audit activities
- Stage 1 Audit: The 1st stage of Initial Certification Audit to determine the adequacy of the client’s management system documentation and readiness to proceed to the Stage 2 audit.
- Stage 2 Audit: The 2nd stage of Initial Certification Audit to evaluate the implementation, including effectiveness, of the client’s management system.
- Recommendation: Upon successful completion of audit activities, Audit Team Leader will recommend to Technical & Certification Committee for further certification process
- Certification Issuance: Technical & Certification Committee will review and approve the certification application based on respective standard requirement. Upon satisfaction, ISO Certificate will be granted
- Surveillance Audit: Annual audit based on respective standard to ensure ongoing compliance.
- Recertification Audit: Renewal of certification is subject to a satisfactory recertification audit carry out before the expiry of the certificate.
How long does it take to be certified with ISO 9001?
The time required for certification can vary widely depending on the size and complexity of the organization, its existing processes, and its commitment to meeting ISO 9001 requirements.
Kindly contact us further at admin@leadercb.com for further information
How much the cost of ISO 9001 certification?
The cost of ISO 9001 certification varies depending on factors such as the size and complexity of the organization, scope of activities and the location.
Kindly contact us further at admin@leadercb.com for further information
I am already certified with ISO 9001 by another Certification Body, can I transfer my certification to LEADER?
Yes, it is possible.
Kindly contact us further at admin@leadercb.com for further information
What is ISO 14001?
ISO 14001 is an internationally recognized standard that sets out the criteria for an environmental management system (EMS). It helps organizations of all sizes and industries to systematically manage and reduce their environmental impact, comply with environmental regulations, and demonstrate a commitment to environmental sustainability.
What are the benefits of ISO 14001?
The benefits of implementing ISO 14001 include:
- Environmental Performance Improvement: Organizations can systematically identify, manage, and reduce their environmental impact, leading to improved environmental performance.
- Cost Savings: Efficient resource use and waste reduction can lead to cost savings.
- Legal and Regulatory Compliance: ISO 14001 helps organizations stay compliant with environmental laws and regulations.
- Enhanced Reputation: Certification can improve an organization’s reputation by demonstrating its commitment to environmental responsibility.
- Competitive Advantage: ISO 14001 certification can be a differentiator in the market and open up new business opportunities.
- Stakeholder Confidence: Customers, investors, and other stakeholders often have greater confidence in organizations with ISO 14001 certification.
Who shall apply ISO 14001 Certification?
ISO 14001 certification is relevant and applicable to any organization, regardless of its size or industry, that wants to establish an effective environmental management system. This includes manufacturing companies, service providers, government agencies, non-profit organizations, and more. The standard can be tailored to meet the specific needs and environmental aspects of each organization, making it versatile and widely applicable. Organizations that are committed to reducing their environmental impact and improving their environmental performance often seek ISO 14001 certification to formalize their efforts.
What is the involved certification process to be certified with ISO 14001?
The certification process based on ISO/IEC 17021-1 requirement involves the following steps:
- Certification Application: Authorized representative of the company require to fill up Certification Application for Application Review purpose and further preparation of Quotation and Certification Agreement
- Quotation and Certification Agreement: Upon acceptance on the terms and conditions, LEADER will proceed with the preparation of Audit Programme and planning of audit activities
- Stage 1 Audit: The 1st stage of Initial Certification Audit to determine the adequacy of the client’s management system documentation and readiness to proceed to the Stage 2 audit.
- Stage 2 Audit: The 2nd stage of Initial Certification Audit to evaluate the implementation, including effectiveness, of the client’s management system.
- Recommendation: Upon successful completion of audit activities, Audit Team Leader will recommend to Technical & Certification Committee for further certification process
- Certification Issuance: Technical & Certification Committee will review and approve the certification application based on respective standard requirement. Upon satisfaction, ISO Certificate will be granted
- Surveillance Audit: Annual audit based on respective standard to ensure ongoing compliance.
- Recertification Audit: Renewal of certification is subject to a satisfactory recertification audit carry out before the expiry of the certificate.
How long does it take to be certified with ISO 14001?
The time required for certification can vary widely depending on the size and complexity of the organization, its existing processes, and its commitment to meeting ISO 14001 requirements.
Kindly contact us further at admin@leadercb.com for further information
How much the cost of ISO 14001 certification?
The cost of ISO 14001 certification varies depending on factors such as the size and complexity of the organization, scope of activities and the location.
Kindly contact us further at admin@leadercb.com for further information
I am already certified with ISO 14001 by another Certification Body, can I transfer my certification to LEADER?
Yes, it is possible.
Kindly contact us further at admin@leadercb.com for further information
What is ISO 13485?
ISO 13485 is an international standard that specifies the requirements for a quality management system specific to the medical device industry. It outlines the processes and procedures necessary to ensure the quality, safety, and effectiveness of medical devices throughout their lifecycle, from design and development to production, distribution, and post-market surveillance.
What are the benefits of ISO 13485?
The benefits of implementing ISO 13485 include:
- Enhanced Product Quality: ISO 13485 helps ensure that medical devices meet stringent quality and safety standards.
- Compliance with Regulations: It assists organizations in complying with regulatory requirements, including Malaysia’s Medical Device Act 2012 and Regulations 2012.
- Improved Efficiency: The standard promotes process efficiency and risk management, leading to better resource utilization.
- Enhanced Customer Confidence: ISO 13485 certification can instil confidence in customers, regulatory bodies, and stakeholders.
- Competitive Advantage: Certification can be a competitive differentiator in the medical device market.
- Global Market Access: ISO 13485 is recognized internationally, facilitating access to global markets.
Who shall apply ISO 13485 Certification?
ISO 13485 certification is relevant for organizations involved in the design, development, production, installation, and servicing of medical devices. This includes manufacturers of medical devices, suppliers of components or services to the medical device industry, and organizations involved in the distribution and servicing of medical devices.
In the context of compliance against Section 15 of Medical Device Act 2012 by Manufacturer, ISO 13485 certification is a mandatory and act as pre-requisite requirement to apply for Establishment License.
What is the involved certification process to be certified with ISO 13485?
The certification process based on ISO/IEC 17021-1 requirement involves the following steps:
- Certification Application: Authorized representative of the company require to fill up Certification Application for Application Review purpose and further preparation of Quotation and Certification Agreement
- Quotation and Certification Agreement: Upon acceptance on the terms and conditions, LEADER will proceed with the preparation of Audit Programme and planning of audit activities
- Stage 1 Audit: The 1st stage of Initial Certification Audit to determine the adequacy of the client’s management system documentation and readiness to proceed to the Stage 2 audit.
- Stage 2 Audit: The 2nd stage of Initial Certification Audit to evaluate the implementation, including effectiveness, of the client’s management system.
- Recommendation: Upon successful completion of audit activities, Audit Team Leader will recommend to Technical & Certification Committee for further certification process
- Certification Issuance: Technical & Certification Committee will review and approve the certification application based on respective standard requirement. Upon satisfaction, ISO Certificate will be granted.
- Surveillance Audit: Annual audit based on respective standard to ensure ongoing compliance.
- Recertification Audit: Renewal of certification is subject to a satisfactory recertification audit carry out before the expiry of the certificate.
How long does it take to be certified with ISO 13485?
The time required for certification can vary widely depending on the size and complexity of the organization, its existing processes, and its commitment to meeting ISO 13485 requirements.
Kindly contact us further at admin@leadercb.com for further information
How much the cost of ISO 13485 certification?
The cost of ISO 13485 certification varies depending on factors such as the size and complexity of the organization, scope of activities and the location.
Kindly contact us further at admin@leadercb.com for further information
I am already certified with ISO 13485 by another Certification Body, can I transfer my certification to LEADER?
Yes, it is possible.
Kindly contact us further at admin@leadercb.com for further information
ISO 45001 is an international standard that specifies requirements for an occupational health & safety (OHS) management system. It provides a framework for organisation to manage risks and opportunities to help prevent worker illness and injuries
Medical Device Conformity Assessment (Technical Documentation) follows requirement imposed in:
- Medical Device Act 2012
- Medical Device Regulations 2012
- Medical Device Regulations 2019
- MDA/GD/0031 Conformity Assessment for Medical Device
- Circular Letter of The Medical Device Authority No. 2 Year 2014 Conformity Assessment Procedures for Medical Device Approved by Recognised Countries
- MDA/GL/08 Guideline for Re-registration of Registered Medical Device
Kindly contact us further at admin@leadercb.com for further information.
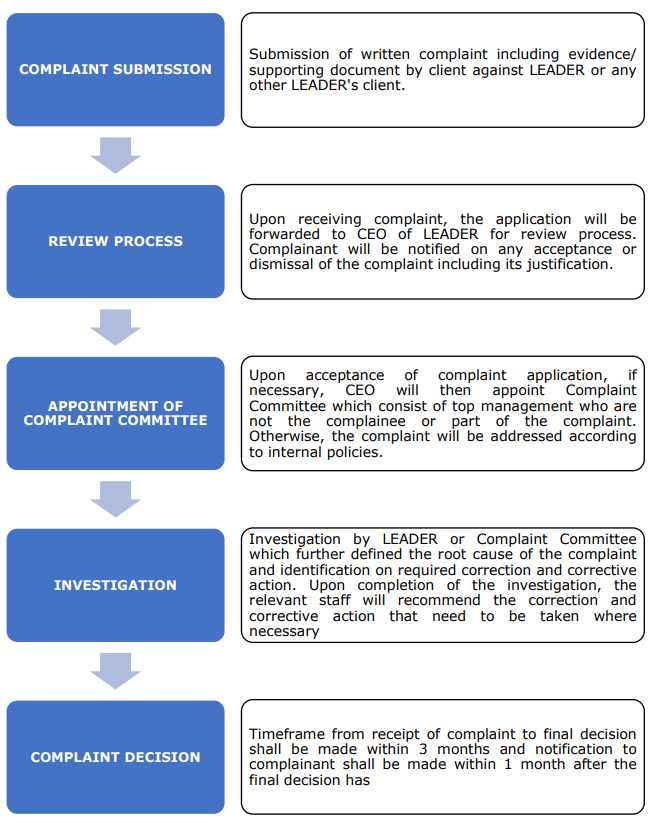
Appeal Process
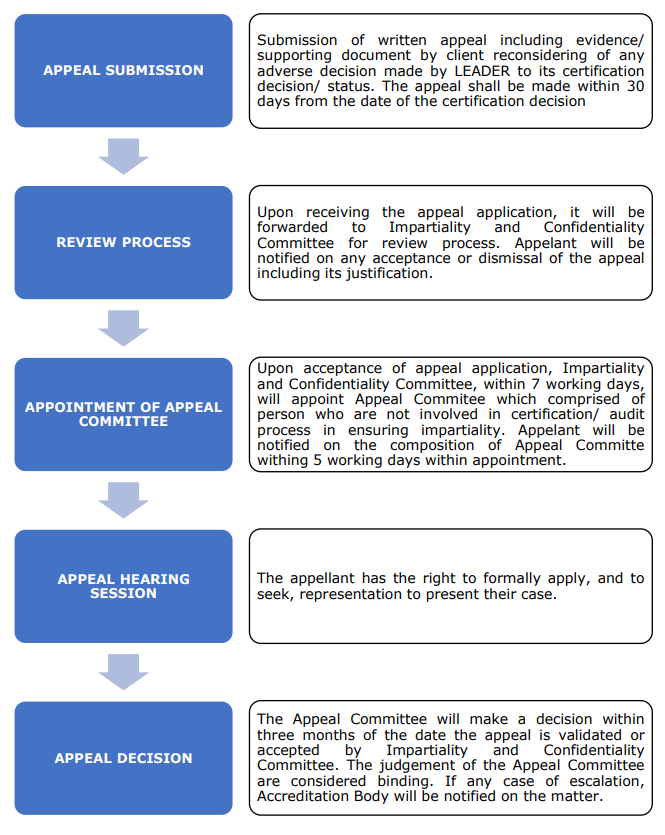
Complaint Process